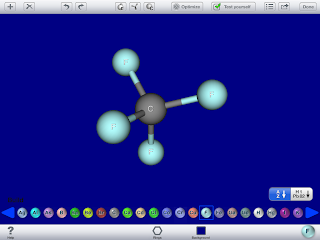
My original plan was to use screen casting apps to have my Organic Chemistry Lab Students create "electronic" lab reports. At the end of this post I will show you an example of one of the first efforts to do just that. But I have found more success in my General Chemistry class using the apps to help the students learn content, specifically molecular shapes. Today we used the app "Mols Editor"
to draw the VSEPR or "Valence shell electron pair repulsion" model of molecular structure. The properties and function of a substance is determined by its molecular structure. The shape is a significant contributor to both structure and function. So it is critical that students develop the ability to look at the formula of a molecule and determine its three dimensional shape. There are several apps available that allow one to build molecules and also see its shape in three dimensions. Many of these apps are not easy to use when constructing molecules. Others have big molecules and beautiful pictures of them that can be rotate but no building function. I just wanted a simple app that allows us to build small molecules to look for specific shapes predicted by VSEPR theory such as linear, trigonal planer, tetrahedral etc. After much searching I found it in Mols Editor. With just a few taps on the screen one can build a structure and it is in the correct orientation. Here is an example of building CF4 :
As a chemist knows carbon can only have four bonds in a molecule like the one above. So each time an atom is "tapped" another atom is bonded to it. However, the app will not let the student create more bonds for an atom than is chemically possible. So in the picture above we see the tetrahedral structure of many carbon compounds. If the central carbon atom is tapped again the student gets a message box that tells the student that no more bonds can be made with that atom. What is really good about this app is it lets students rotate the molecule with their finger and they can see the molecule from different angles. I sent my students a quick survey on google forms asking them to rate the app for me. They were all very positive in that it helped them see the structure. The one downside is that the app does not provide the bond angles. I really think this experience helped my students get a good foundation in VSEPR structures and I plan to use it again.
Small steps towards a digital lab report
As I mentioned, I wanted to have my Organic Chemistry students create lab reports that are digital using multimedia presentations. The screencasting apps seemed like a very good tool to do this. I gave my students the option of doing the traditional hand-written lab report or going electronic. Only two students took me up on the offer to go digital. I was surprised by this. I think the hand written form is much harder. I expect them to write a 1 1/2 to 2 page discussion with much deep analysis of the chemical concepts applied to the practical aspect of the experiment. Nevertheless, 13 out of 15 chose to write. I need to figure this out! Maybe they are just fearful of the unknown. Maybe they are not sure of my expectations. Maybe they are worried about their grade. I need to have an honest talk with them.
I must first say that these are top-notch students. I have had many of them in previous classes. And I know their writing quite well. This group is a selection of very good technical writers. I have no problem if they write a few less lab reports in order to try to verbally explain their analysis in a screencast. I just have to figure out how to get them over the threshold.
Here is one of the two "digital lab reports" I received today:

No comments:
Post a Comment