Molecular Orbital Modeling with Mols Editor
There are so many apps out there for modeling molecules. For my General Chemistry class I want to build simple molecules and be able to look at them three-dimensionally. I also want to be able to look at the molecular orbital contour diagrams. So far the best app for this has been Mols Editor. In previous posts I have shown the basic 3-D structure. Now I want my students to draw those crazy contour diagrams. These are hard to visualize and draw because they are so abstract and 3-D on top of that. Mols editor lets you build the molecule and then there is an button to display the molecular orbitals. Here is a picture of methane CH4.
You can see result of the SP3 hybridization of carbon and the overlap with the hydrogen 1s orbitals. My students found this very helpful. Of course even harder to draw and visualize are the double and triple bonded molecules. Here is an example of ethene C2H6.
Bond Angles and R/S configuration and Spectra with iSpartan
I also want to show my students the bond angles. Unfortunately Mols editor does not yet display bond angles. At least I have not seen that yet. But another app called iSpartan lets you draw the molecule and then it renders the molecule in 3-D. It also allows you to analyze bond angles, R and S configuration and it shows the NMR and Infrared spectra. Here is an example of finding the bond angle.
When you highlight three connected atoms (circled in above photo) the app automatically calculates the bond angle. As you can see in the picture of CBr4, which is tetrahedral, the bond angle is 109.5 which is correct. I figured this app out a little too late for my current General Chemistry students, but maybe I will use it next semester.
If a particular atom is chiral the app tells if the arrangement is the R or S. This is of particular interest to my Organic Chemistry students. Here is a screenshot of bromo-chloro-fluro-iodomethane. And you can see it is the S-configuration of it.
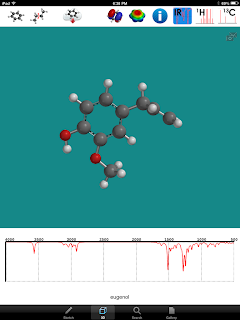
The last thing I want to point out about iSpartan is that you can also obtain the spectra of various molecules if they are in the available database. Next week we are doing the steam distillation lab of cloves. We will extracting Eugenol. I am happy to say that this molecule is in the data base. So my students can run the IR on their extracted sample and then compare it to the one on the app.
As you can see in the upper left corner, H-NMR and C-NMR also are available.
So for me, Mols Editor and iSpartan are two of the best apps for molecular modeling. There are others that I will "review" later. The one downside of iSpartan is the cost. Currently it runs around $20. Mols Editor has three versions. One is free. The one I am currently using is $1.99. I find it to be quite adequate. The third version has self-testing. It costs $4.99. I have not been able to find that as useful as I had hoped to yet. But these two apps really, to me anyway, really enhance my job of teaching a science that is 3-dimensional. Chemistry teaching will never be the same once this type of tool catches on!




I know this article is several years old, but I am very interested in the Mols Editor app. Will you post a link to this....can't seem to find it.
ReplyDeletethanks
Hi, did you find the link?
ReplyDeleteThanks